OmegaXL by Great HealthWorks
OmegaXL® – Over 15,000+ FIVE STAR Reviews
New Ways to BUY and SAVE
New! BOGO
With
SleepXL


$119.97
online retailers
$49.90
Bundle Offer
First Order Price on Auto-ship
BUY NOW
Bundle
Best
Value


$119.99
online retailers
$49.90
Bundle Offer
First Order Price on Auto-ship
BUY NOW

OmegaXL® Auto-Ship, 60 Day Money Back Guarantee
For OmegaXL only, if you are not satisfied with your auto-ship purchase, cancel your auto-ship within 60 days of your first purchase date. Read More.
OmegaXL® One-Time Purchase Standard Return Policy
For one-time purchases of OmegaXL, return the entire purchase of unused, sealed bottles within 30 days for a refund (minus shipping & handling). Any free bottles, must be returned. Read More.

Meet Our Brand Ambassadors
MUSSELS
FOR YOUR
JOINTS
SUSTAINABLY SOURCED FROM NEW ZEALAND GREEN-LIPPED MUSSELS
OmegaXL achieved an environmental milestone years ago, with the New Zealand Department of Fisheries, ensuring the eco-sustainability of the New Zealand Green-Lipped Mussel. Our farming process is both eco-friendly and sustainable.


VALUE SIZE
SAVINGS
300CT
$219.99
On-line Retailers
$194.95
Bulk Buy Value
This is a one time purchase

Sleep Like a Baby
Wake Refreshed & Rested*

$57.90
Online Retailers
$39.95
2 bottle, Auto-ship
$24.95
1 bottle, Auto-ship
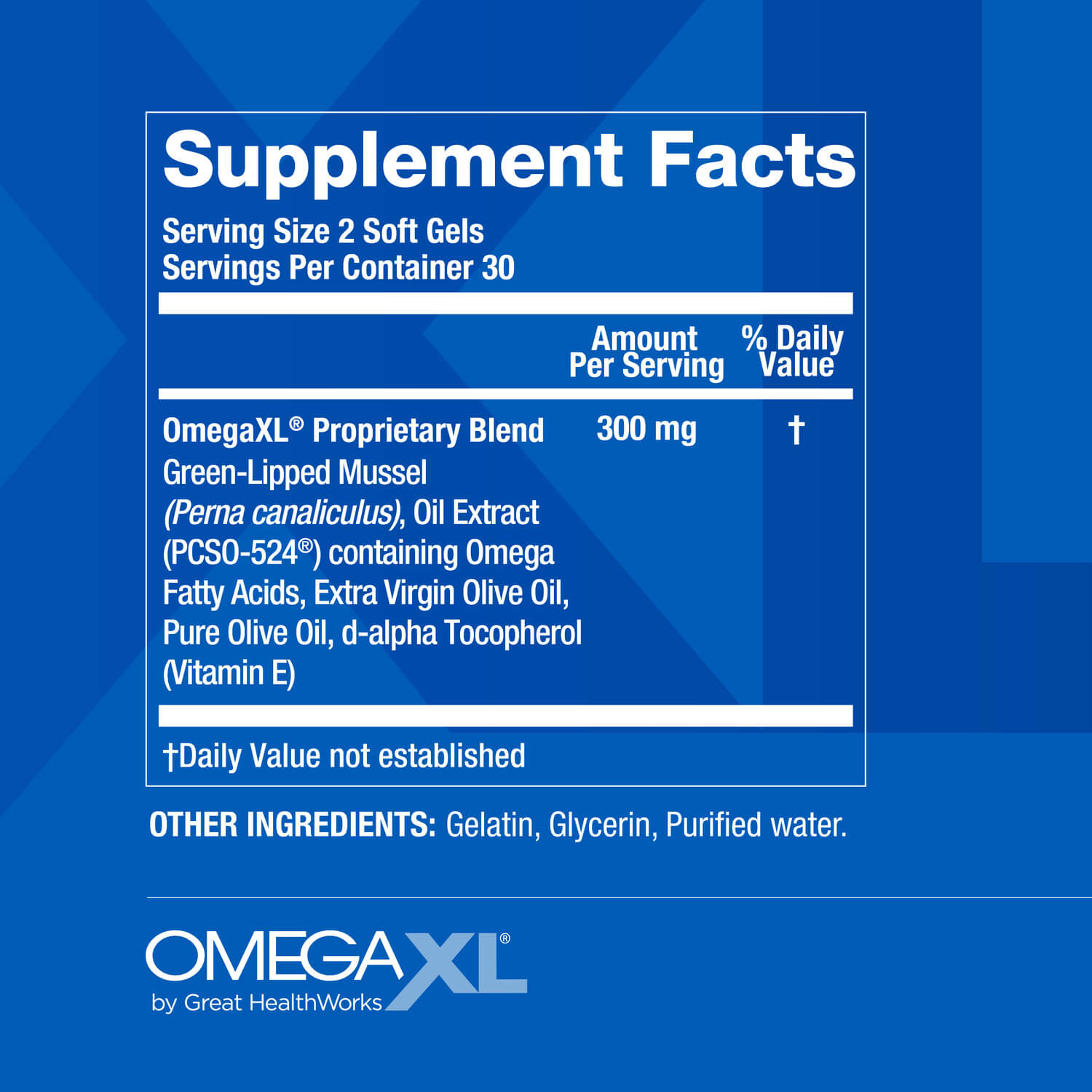
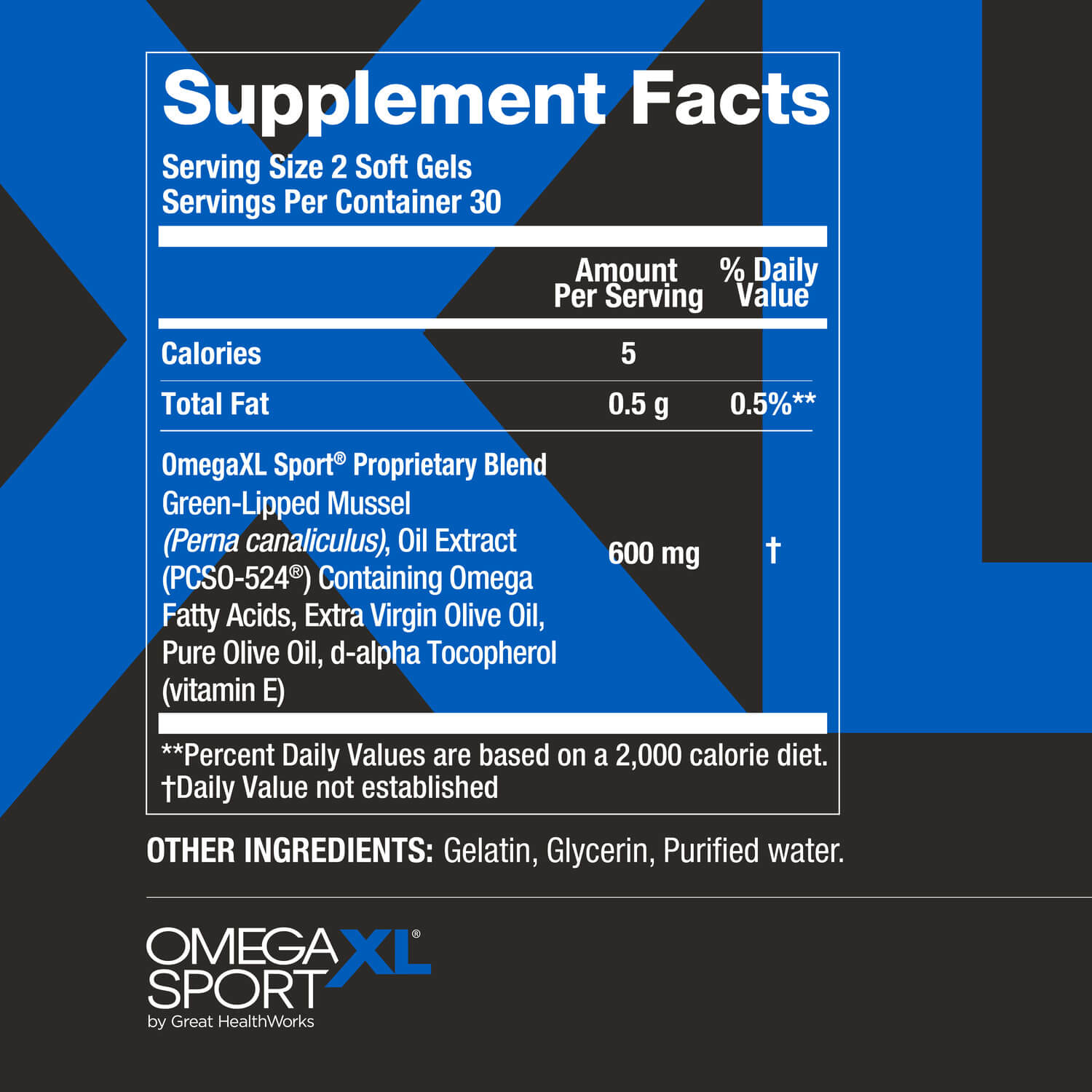
See supplement facts
SUPPORTS BONE HEALTH,
IMMUNE SYSTEM FUNCTION*

$9.99
online retailers
$6.95
Save up to 30%
with Auto-ship
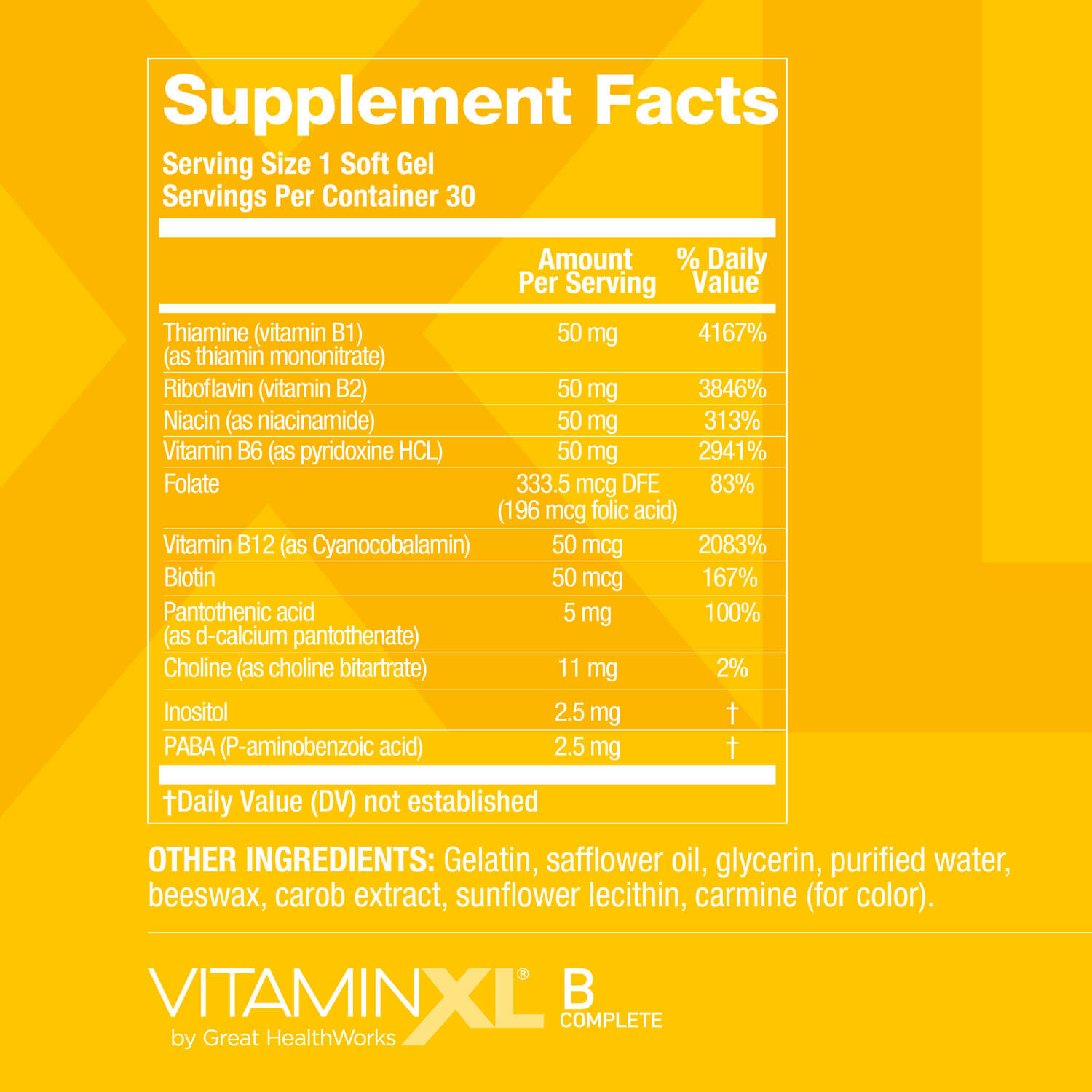
See supplement facts
NEW!
Essential immune support*

$24.99
On-line retailers
$15.95
Save up to 30%
with Auto-ship
See supplement facts
PROMOTE GUT BALANCE WITH TRIPLE-ACTION FORMULA*

$23.99
On-line retailers
$15.95
Save up to 30%
with Auto-ship
See supplement facts
OMEGA-7 SUPPLEMENT TO HELP PROMOTE HEART HEALTH*

$21.99
online retailers
$13.95
Save up to 30%
with Auto-ship
See supplement facts
NEW!
B ENERGETIC*, B GREAT

$19.99
On-line retailers
$12.95
Save up to 30%
with Auto-ship
See supplement facts
A SUPER NUTRIENT
FOR BODY HEALTH*

$21.99
On-line retailers
$13.95
Save up to 30%
with Auto-ship
See supplement facts

Standard return policy for all non-OmegaXL Auto-ship products
If you are not satisfied with your purchase of any Great HealthWorks product, return the entire purchase of unused, sealed bottles, within 30 days of the purchase date to receive a refund of the purchase price (minus any shipping and handling). Read More.